© 2026 JeeHub. All rights reserved.
Feb 1, 2024
JEE Mains
Shift: 2
Total Questions Available: 90
Question 1

Options:
A)
A : n-Pentane B : Cis - 2 - buteneB)
A : 2-Pentyne B : Cis-2-buteneC)
A : n-Pentane B : trans-2-buteneD)
A : 2-Pentyne B : trans-2-buteneQuestion 2
Options:
A)
B)
C)
D)
Question 3
Options:
A)
Inner orbital Complex, Spin paired ComplexB)
Spin paired Complex, Spin free ComplexC)
Spin free Complex, Spin paired ComplexD)
Outer orbital Complex, Inner orbital ComplexQuestion 4
| List I (Reactants) | List II (Product) |
|---|---|
| (A) Phenol, Zn/Δ | (I) Salicylaldehyde |
| (B) Phenol, CHCl3, NaOH, HCl | (II) Salicylic acid |
| (C) Phenol, CO2, NaOH, HCl | (III) Benzene |
| (D) Phenol, Conc. HNO3 | (IV) Picric acid |
Options:
A)
(A)-(IV), (B)-(I), (C)-(II), (D)-(III)B)
(A)-(III), (B)-(I), (C)-(II), (D)-(IV)C)
(A)-(IV), (B)-(II), (C)-(I), (D)-(III)D)
(A)-(III), (B)-(IV), (C)-(I), (D)-(II)Question 5
Options:
A)
B)
C)
D)
Question 6
Options:
A)
B)
C)
D)
Question 7
Options:
A)
3B)
2C)
1D)
4Numerical TypeQuestion 8
Question 9
Options:
A)
2B)
C)
D)
3Question 10
Options:
A)
B)
C)
D)
Question 11
Options:
A)
and both are equivalence relationsB)
Only is an equivalence relationC)
Only is an equivalence relationD)
Neither nor is an equivalence relationQuestion 12
Options:
A)
22B)
17C)
15D)
28Question 13
Options:
A)
B)
C)
D)
Question 14
Options:
A)
5B)
3C)
2D)
0Numerical TypeQuestion 15
Numerical TypeQuestion 16
Question 17
Options:
A)
(B), (C), (D), (E) onlyB)
(A), (C), (D), (E) onlyC)
(A), (B), (C), (E) onlyD)
(A), (B), (C), (D) onlyQuestion 18
Options:
A)
B)
C)
D)
Question 19

Options:
A)
B)
C)
D)
Question 20
Options:
A)
Statement I is false but Statement II is trueB)
Both Statement I and Statement II are falseC)
Both Statement I and Statement II are trueD)
Statement I is true but Statement II is falseQuestion 21
Options:
A)
B)
C)
D)
Question 22
Options:
A)
Both Statement I and Statement II are trueB)
Both Statement I and Statement II are falseC)
Statement I is true but Statement II is falseD)
Statement I is false but Statement II is trueQuestion 23
Options:
A)
Both Statement I and Statement II are falseB)
Both Statement I and Statement II are trueC)
Statement I is false but Statement II is trueD)
Statement I is true but Statement II is falseQuestion 24
Options:
A)
B)
C)
D)
Question 25
Options:
A)

B)
C)
D)
Numerical TypeQuestion 26
Numerical TypeQuestion 27
Numerical TypeQuestion 28
Numerical TypeQuestion 29
Numerical TypeQuestion 30

Question 31
Options:
A)
140B)
175C)
125D)
150Question 32
Options:
A)
2B)
1C)
3D)
Question 33
Options:
A)
18B)
24C)
26D)
36Question 34
Options:
A)
169B)
121C)
225D)
144Question 35
Options:
A)
B)
C)
D)
Question 36
Options:
A)
B)
C)
D)
Question 37
Options:
A)
Statement I is false but Statement II is trueB)
Both Statement I and Statement II are trueC)
Statement I is true but Statement II is falseD)
Both Statement I and Statement II are falseQuestion 38
Options:
A)
Phosphorous and halogens onlyB)
Nitrogen, Sulphur and Phosphorous onlyC)
Nitrogen, Sulphur, phosphorous and halogensD)
Nitrogen and Sulphur onlyNumerical TypeQuestion 39
Numerical TypeQuestion 40
| S.No. | Time /s | Total pressure /(atm) |
|---|---|---|
| 1. | 0 | 0.1 |
| 2. | 115 | 0.28 |
Numerical TypeQuestion 41
Numerical TypeQuestion 42
Question 43
Options:
A)
8B)
9C)
D)
Question 44
Options:
A)
B)
C)
D)
Question 45
Options:
A)
2B)
1C)
D)
Question 46
Options:
A)
0B)
3C)
1D)
2Question 47
Options:
A)
B)
1C)
D)
Question 48
Options:
A)
-1B)
2C)
0D)
1Numerical TypeQuestion 49
Numerical TypeQuestion 50
Numerical TypeQuestion 51
Question 52
| List I (Compound) | List II (Use) |
|---|---|
| (A) Carbon tetrachloride | (I) Paint remover |
| (B) Methylene chloride | (II) Refrigerators and air conditioners |
| (C) DDT | (III) Fire extinguisher |
| (D) Freons | (IV) Non Biodegradable insecticide |
Options:
A)
(A)-(II), (B)-(III), (C)-(I), (D)-(IV)B)
(A)-(III), (B)-(I), (C)-(IV), (D)-(II)C)
(A)-(I), (B)-(II), (C)-(III), (D)-(IV)D)
(A)-(IV), (B)-(III), (C)-(II), (D)-(I)Question 53

Options:
A)
Malonic acidB)
Oxalic acidC)
Succinic acidD)
Gluconic acidQuestion 54
Options:
A)
(A) is true but (R) is falseB)
Both (A) and (R) are true and (R) is the correct explanation of (A)C)
Both (A) and (R) are true but (R) is not the correct explanation of (A)D)
(A) is false but (R) is trueQuestion 55
Options:
A)
B)
C)
D)
Question 56
Options:
A)
7B)
9C)
3D)
5Question 57
Options:
A)
102B)
138C)
132D)
108Question 58
Options:
A)
800B)
890C)
790D)
690Question 59
Options:
A)
B)
C)
D)
Numerical TypeQuestion 60
Numerical TypeQuestion 61
Numerical TypeQuestion 62
Numerical TypeQuestion 63
Numerical TypeQuestion 64
Question 65

Options:
A)
B)
C)
D)
Question 66
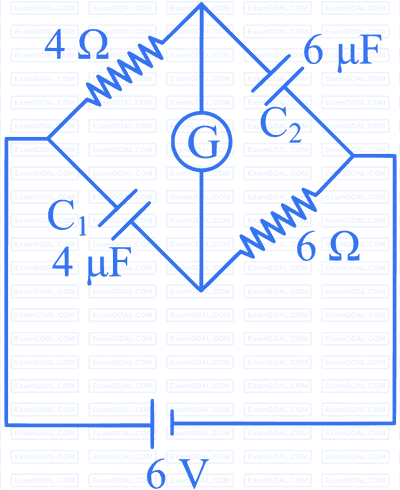
Options:
A)
1B)
C)
D)
Question 67
Options:
A)
thB)
thC)
100 timesD)
10 timesQuestion 68
Options:
A)
B)
C)
D)
Numerical TypeQuestion 69
Numerical TypeQuestion 70
Numerical TypeQuestion 71
Numerical TypeQuestion 72
Numerical TypeQuestion 73
Question 74
Options:
A)
30B)
24C)
12D)
25Question 75
| List I (Number) | List II (Significant figure) |
|---|---|
| (A) 1001 | (I) 3 |
| (B) 010.1 | (II) 4 |
| (C) 100.100 | (III) 5 |
| (D) 0.0010010 | (IV) 6 |
Options:
A)
(A)-(II), (B)-(I), (C)-(IV), (D)-(III)B)
(A)-(IV), (B)-(III), (C)-(I), (D)-(II)C)
(A)-(I), (B)-(II), (C)-(III), (D)-(IV)D)
(A)-(III), (B)-(IV), (C)-(II), (D)-(I)Question 76
Options:
A)
B)
C)
D)
Question 77
Options:
A)
B)
C)
D)
Numerical TypeQuestion 78
Numerical TypeQuestion 79
Numerical TypeQuestion 80
Numerical TypeQuestion 81
Question 82
Options:
A)
B)
C)
D)
Question 83
Train A is moving along two parallel rail tracks towards north with speed and train B is moving towards south with speed . Velocity of train B with respect to A and velocity of ground with respect to B are (in ):
Options:
A)
-50 and -30B)
-50 and 30C)
-30 and 50D)
50 and -30Question 84
Options:
A)
B)
C)
D)
Question 85
Options:
A)
B)
C)
D)
Question 86
Options:
A)
1.0B)
1.5C)
2.0D)
0.5Question 87
Options:
A)
2.5B)
5C)
10D)
2Question 88

Options:
A)
B)
C)
D)
Question 89
Options:
A)
11B)
13C)
15D)
21Numerical TypeQuestion 90
