© 2026 JeeHub. All rights reserved.
Apr 15, 2023
JEE Mains
Shift: 1
Total Questions Available: 72
Question 1
Options:
A)
It has a fairly high melting point.B)
It is associated with more than one values.C)
It has high solubility in benzene.D)
It is a crystalline solid.Question 2

Options:
A)
B)
bC)
D)
Numerical TypeQuestion 3
Question 4
Options:
A)
-21B)
21C)
19D)
0Question 5
Options:
A)
4B)
3C)
5D)
6Question 6
Options:
A)
30B)
40C)
25D)
35Question 7
Options:
A)
306B)
304C)
308D)
302Numerical TypeQuestion 8
Numerical TypeQuestion 9
Question 10
Options:
A)
B)
C)
D)
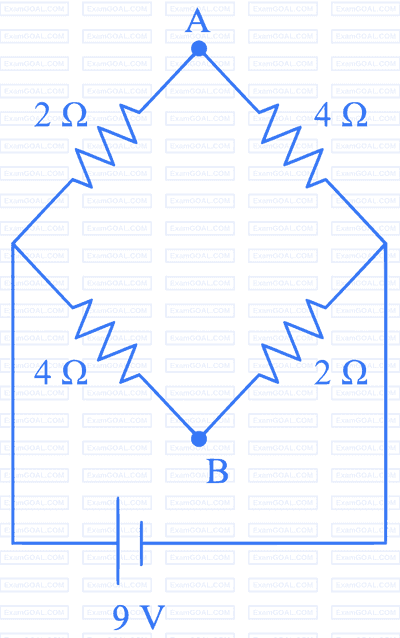
Question 11
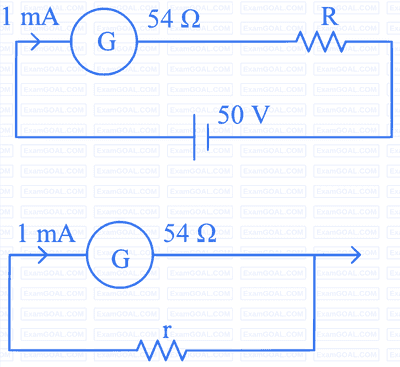
Options:
A)
andB)
(A) and (B)C)
(C) and (E)D)
(C) and (D)Question 12

Options:
A)
B)
ZeroC)
D)
Question 13
Options:
A)
Statement I is true but Statement II is falseB)
Both Statement I and Statement II are trueC)
Both Statement I and Statement II are falseD)
Statement I is false but Statement II is trueNumerical TypeQuestion 14

Numerical TypeQuestion 15

Numerical TypeQuestion 16
Question 17
Options:
A)
water is evaporated in presence of catalyst.B)
carbon monoxide is oxidized to carbon dioxide.C)
carbon is oxidized to carbon monoxide.D)
carbon dioxide is reduced to carbon monoxide.Question 18
Options:
A)
B)
C)
D)
Question 19
Options:
A)

B)

C)

D)

Question 20
Options:
A)
Water present in the mobile phase gets absorbed by the paper which then forms the stationary phase.B)
Water present in the pores of the paper forms the stationary phase.C)
Paper sheet forms the stationary phase.D)
Paper and water present in its pores together form the stationary phase.Question 21

Options:
A)

B)

C)

D)

Question 22
Options:
A)
(C) and (D) are correctB)
(A) and (B) are correctC)
(B) and (C) are correctD)
(A) and (D) are correctQuestion 23

Options:
A)

B)

C)

D)

Numerical TypeQuestion 24
Numerical TypeQuestion 25
Numerical TypeQuestion 26
Numerical TypeQuestion 27
Question 28
Options:
A)
3B)
6C)
2D)
11Question 29
Options:
A)
B)
C)
D)
Question 30
Options:
A)
36B)
27C)
42D)
30Numerical TypeQuestion 31
Question 32
Options:
A)
B)
C)
D)
Question 33
Options:
A)
B)
C)
D)
Question 34
Options:
A)
B)
2C)
6D)
Question 35
Options:
A)
B)
C)
D)
Numerical TypeQuestion 36
Numerical TypeQuestion 37
Numerical TypeQuestion 38
Numerical TypeQuestion 39
Numerical TypeQuestion 40
Question 41
Options:
A)
Both Statement I and Statement II are incorrectB)
Statement I is correct but Statement II is incorrectC)
Both Statement I and Statement II are correctD)
Statement I is incorrect but Statement II is correctQuestion 42
Options:
A)
B)
(B), (C)C)
(B), (C), (D)D)
(A), (D)Numerical TypeQuestion 43
Numerical TypeQuestion 44
Numerical TypeQuestion 45
Question 46
Options:
A)
B)
C)
D)
3Question 47
Options:
A)
11B)
12C)
13D)
14Question 48
Options:
A)
B)
C)
D)
Numerical TypeQuestion 49
Question 50
Options:
A)
B)
C)
D)
Question 51
Options:
A)
B)
C)
D)
Question 52
Options:
A)
B)
(C) and (D) onlyC)
(A), (B) and (C) OnlyD)
(A), (C) and (D) onlyQuestion 53
Options:
A)
B)
C)
D)
Question 54
| List I | List II |
|---|---|
| (A) Microwave | (I) to |
| (B) Ultraviolet | (II) to |
| (C) X-Ray | (III) to |
| (D) Infra-red | (IV) to |
Options:
A)
(A)-(I), (B)-(IV), (C)-(II), (D)-(III)B)
(A)-(IV), (B)-(I), (C)-(II), (D)-(III)C)
(A)-(IV), (B)-(I), (C)-(III), (D) -(II)D)
(A)-(IV), (B)-(II), (C)-(I), (D)-(III)Question 55
Options:
A)
B)
C)
D)
Question 56
Options:
A)
B)
C)
D)
Question 57

Options:
A)
(i) , (ii) , (iii) , (iv)B)
(i) , (ii) , (iii) , (iv) , (v)C)
(i) , (ii) , (iii) , (iv) , (v)D)
(i) , (ii) , (iii) , (iv)Question 58

Options:
A)

B)

C)
D)

Numerical TypeQuestion 59
Question 60
Options:
A)
21B)
22C)
18D)
20Question 61
Options:
A)
-2B)
4C)
-4D)
2Question 62
Options:
A)
72B)
54C)
45D)
63Question 63
Options:
A)
15B)
8C)
6D)
12Numerical TypeQuestion 64
Numerical TypeQuestion 65
Numerical TypeQuestion 66
Question 67
Options:
A)
B)
C)
D)
Question 68
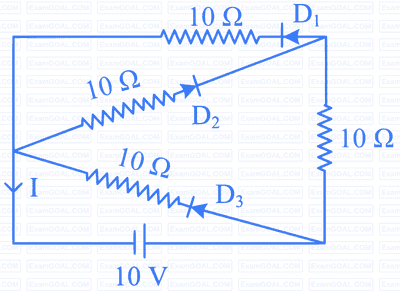
Options:
A)
1AB)
C)
D)
Question 69
Options:
A)
Positive - axisB)
Positive - axisC)
Positive - axisD)
In - planeQuestion 70
Options:
A)
B)
2C)
39.9D)
1Numerical TypeQuestion 71
Numerical TypeQuestion 72