© 2026 JeeHub. All rights reserved.
Jan 31, 2023
JEE Mains
Shift: 2
Total Questions Available: 72
Question 1
Options:
A)
B)
C)
D)
Question 2
Options:
A)
A, B and D onlyB)
A and C onlyC)
B and D onlyD)
B, D and E onlyQuestion 3
Options:
A)
is equal to 9B)
is equal toC)
does not existD)
is equal to 27Question 4
Options:
A)
B)
C)
D)
Numerical TypeQuestion 5
Question 6
Options:
A)
2.25RB)
C)
D)
Question 7
| LIST I | LIST II | ||
|---|---|---|---|
| A. | Microwaves | I. | Physiotherapy |
| B. | UV rays | II. | Treatment of cancer |
| C. | Infra-red light | III. | Lasik eye surgery |
| D. | X-ray | IV. | Aircraft navigation |
Options:
A)
A - IV, B - III, C - I, D - IIB)
A - II, B - IV, C - III, D - IC)
A - III, B - II, C - I, D - IVD)
A - IV, B - I, C - II, D - IIIQuestion 8
Options:
A)
B)
C)
HD)
Question 9
Options:
A)
B)
C)
D)
Question 10
| LIST I | LIST II | ||
|---|---|---|---|
| A. | Angular momentum | I. | |
| B. | Torque | II. | |
| C. | Stress | III | |
| D. | Pressure gradient | IV. | |
Options:
A)
A - I, B - IV, C - III, D - IIB)
A - III, B - I, C - IV, D - IIC)
A - IV, B - II, C - I, D - IIID)
A - II, B - III, C - IV, D - IQuestion 11
Options:
A)
B and D onlyB)
A and onlyC)
and onlyD)
A and E onlyQuestion 12
Options:
A)
Both (A) and (R) are true and (R) is the correct explanation of (A)B)
(A) is true but (R) is falseC)
Both (A) and (R) are true but is not the correct explanation of (A)D)
(A) is false but (R) is trueNumerical TypeQuestion 13
Numerical TypeQuestion 14
Question 15
Options:
A)
B)
C)
D)
Question 16
Options:
A)
two solutions and both are negativeB)
two solutions and only one of them is negativeC)
four solutions two of which are negativeD)
no solutionQuestion 17
Options:
A)
B)
C)
D)
Question 18
Options:
A)
4B)
2C)
D)
Question 19
Options:
A)
B)
C)
D)
Numerical TypeQuestion 20
Question 21
Options:
A)
B)
C)
D)
Question 22
Options:
A)
B)
C)
D)
Question 23
Options:
A)
0.3B)
0.2C)
0.5D)
0.4Question 24
Options:
A)
B)
C)
D)
Question 25
Options:
A)
B)
C)
D)
Question 26
Options:
A)
B)
30C)
D)
Question 27
Options:
A)

B)

C)

D)

Question 28
Options:
A)

B)

C)

D)

Question 29
Options:
A)

B)

C)

D)

Question 30
Options:
A)
B)
C)
D)
Question 31
Options:
A)
Both Statement I and Statement II are falseB)
Both Statement I and Statement II are rueC)
Statement I is false but Statement II is trueD)
Statement I is true but Statement II is falseQuestion 32
Options:
A)
GammaxeneB)
ChloropicrinC)
ChloralD)
Freon-12Numerical TypeQuestion 33
Numerical TypeQuestion 34
Numerical TypeQuestion 35
Numerical TypeQuestion 36
Question 37
Options:
A)
336B)
449C)
339D)
560Question 38
Options:
A)
450B)
900C)
650D)
500Question 39
Options:
A)
24B)
C)
9D)
Question 40
Options:
A)
B)
C)
D)
Question 41
Options:
A)
B)
C)
D)
Question 42
Options:
A)
64B)
C)
32D)
Numerical TypeQuestion 43
Numerical TypeQuestion 44
Numerical TypeQuestion 45
Question 46
Options:
A)
B)
C)
D)
Question 47
Options:
A)
Metal B will not emit photo-electronsB)
Both metals and will not emit photo-electronsC)
Metal A will not emit photo-electronsD)
Both metals A and B will emit photo-electronsQuestion 48
Options:
A)
B)
C)
D)
Question 49
Options:
A)
B)
C)
D)
Question 50
Options:
A)
Copper oxideB)
Copper gauzeC)
D)
Question 51
Options:
A)
hexa-1, 3, 5-trieneB)
cyclohexa-1, 4-dieneC)
cyclohexa - 1,3 - dieneD)
1-methylcyclopenta-1, 4-dieneQuestion 52
Options:
A)
Phenolphthalein is a suitable indicator for a weak acid vs strong base titration.B)
Methyl orange may be used for a weak acid vs weak base titration.C)
Methyl orange is a suitable indicator for a strong acid vs weak base titration.D)
Phenolphthalein may be used for a strong acid vs strong base titration.Numerical TypeQuestion 53
Numerical TypeQuestion 54
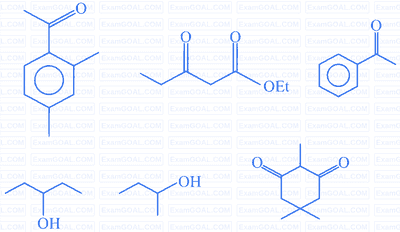
Numerical TypeQuestion 55
Question 56
Options:
A)
is transitive but is notB)
both and are symmetricC)
neither nor is transitiveD)
is symmetric but is notQuestion 57
Options:
A)
B)
3C)
2D)
Numerical TypeQuestion 58
Numerical TypeQuestion 59
Question 60
Options:
A)
Net potential of the system cannot be zero at a point but net electric field can be zero at that pointB)
Net potential of the system at a point can be zero but net electric field can't be zero at that point.C)
Both the net potential and the net electric field cannot be zero at a point.D)
Both the net potential and the net field can be zero at a point.Question 61
Options:
A)
B)
C)
D)
Question 62
Options:
A)
B)
C)
D)
Numerical TypeQuestion 63
Numerical TypeQuestion 64
Numerical TypeQuestion 65
Numerical TypeQuestion 66
Numerical TypeQuestion 67

Numerical TypeQuestion 68
Numerical TypeQuestion 69
Numerical TypeQuestion 70
Numerical TypeQuestion 71
Numerical TypeQuestion 72